- Ensuring clinical success
- Tackling large scale regulatory challenges
- Finding the right framework for reimbursement and market access
- Cross-border healthcare
- Outcome based agreements
- Tackling large scale regulatory challenges
- Patient informed policy decisions
THE FUTURE OF ADVANCED THERAPIES
1,200+
Attendees
200+
Speakers
50+
Exhibitors
50+
Biotech Start-Ups
2
Days
About Advanced Therapies Congress USA
The Advanced Therapies Congress is America's most exciting cell and gene therapy conference and exhibition. The event is designed for the leaders of pioneering ATMP companies and their most senior executives in charge of the latest tech and strategies that are driving the industry forward.
The conference brings together a community of 1,200 global executives at the Boston Convention & Exhibition Center, Boston, to be inspired by 200 speakers and 50 start-ups over 2 incredible days. The Advanced Therapies Congress features speakers from across the entire value chain of cell and gene therapy development; forward-thinking pharma, biotech and start-up companies, researchers, clinicians, academics, HTAs, payers and regulators.
Speaker line-up
President and Chief Scientific Officer
Nanoscope Therapeutics

Sponsors include
What is the Advanced Therapies Congress?

The Advanced Therapies Congress features speakers from across the entire value chain of cell and gene therapy development with 8 tracks of content over 2 days.

50 leading solution providers to meet your needs across all stages of cell and gene therapy development

Connect with thousands of cell and gene therapy experts in 12+ hours of built-in 1-2-1 networking
Attendees include












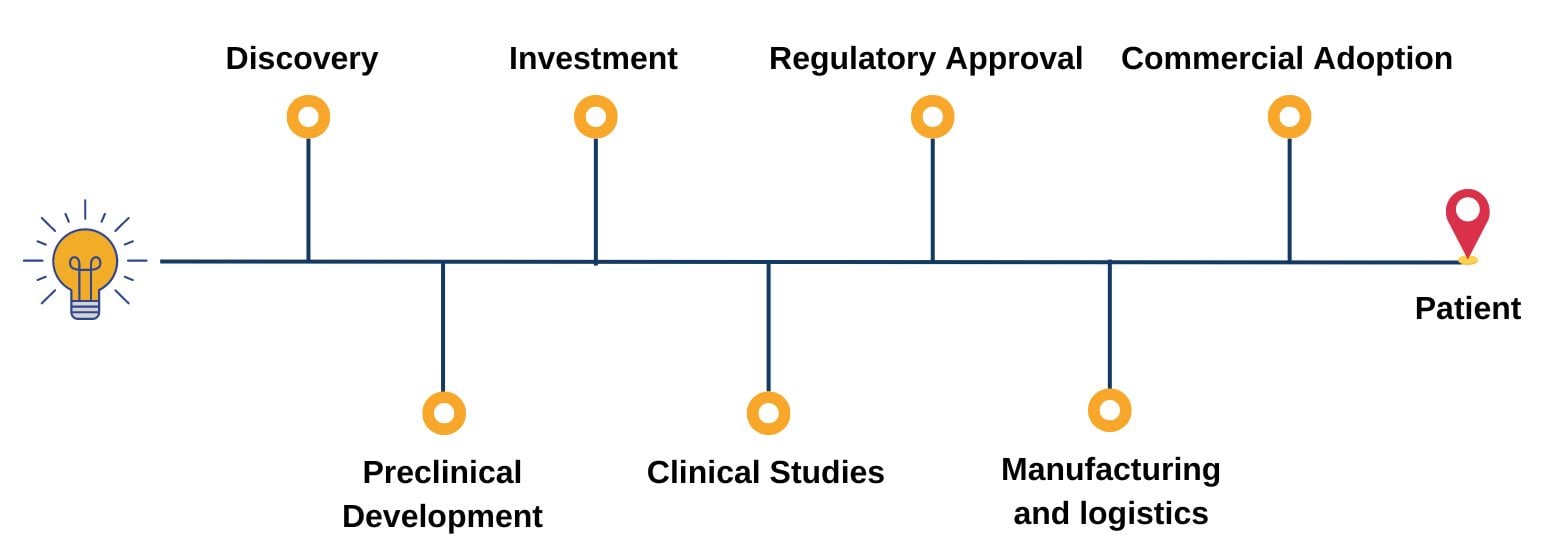
Covering the entire pipeline of ATMPs
The Advanced Therapies Congress brings together speakers from across the entire value chain of cell and gene therapy development; forward-thinking pharma, biotech and startup companies, researchers, clinicians, academics, HTAs, payers, regulators.
America's most exciting commercial cell and gene therapy conference
- Autologous vs allogeneic
- Tackling solid tumours
- Combination therapy
- CAR-T, NK, Gamma-Delta cells
- Patient stratification and precision medicine approaches
- Stem cells
- Regenerative Medicine
- MSCs vs iPSCs
- Cord blood advancements
- Next generation of gene therapy
- Moving from rare to more common diseases
- Tackling immunogenicity
- Rare diseases & orphan drugs
- Novel gene delivery systems
- Gene editing
- Automation & closed systems
- Scaling up to commercial level
- Keeping costs low
- In house vs outsourcing
- Continuous manufacturing
- Scaling up for commercial production
- In house vs outsourcing
- Logistics, transportation, distribution & cold chain
- Moving from clinical to commercial
- Patient experience
- Supply chain
- Point of care delivery
- Latest in academic research
- Latest cutting-edge technologies success

"Very insightful and cutting-edge talks and panel discussions"
– Consultant, ProPharma Group

"Great chance to connect with relevant people from the ecosystem"
– Head of Investment, Govt of Catalonia

"The event attracts a wide range of great people from across the industry. It is a fantastic networking opportunity"
– Senior Scientist, Rinri Therapeutics
Join us in Philadelphia | November 18-19 2025

























































































