Our Story
June 9 - 11, 2026
Thomas M. Menino Convention & Exhibition Center | Boston, MA
The World Orphan Drug Congress USA is the defining event for the rare disease and orphan drug space globally. Widely regarded as the most important event for all rare disease stakeholders to attend, we are yet again bringing together top thought leaders from around the globe for a world-class event on June 9-11, 2026 in Boston, MA.
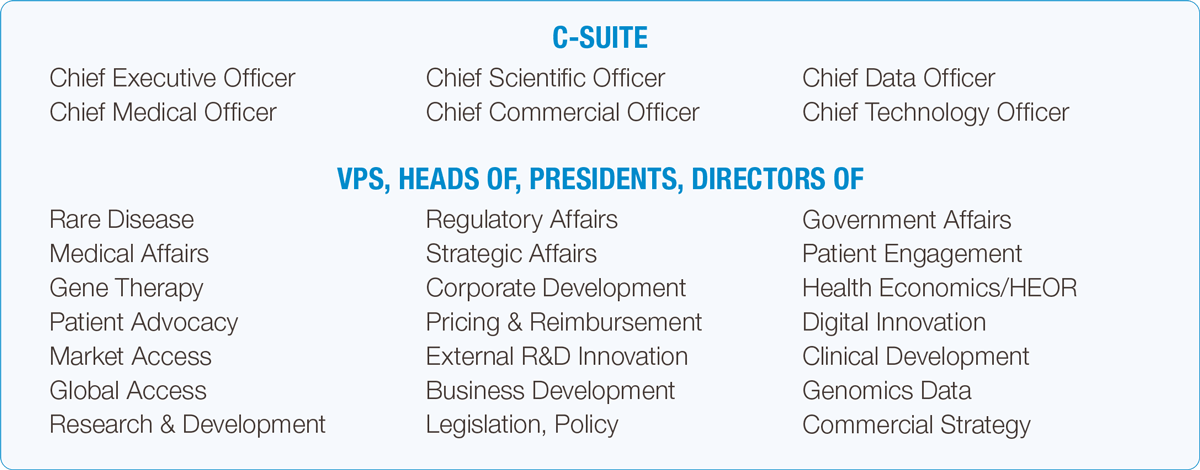
Over 3 days, you will have the opportunity to hear from 300+ speakers across, our 16 themes of content, engage in networking opportunities with over 2,000+ rare disease attendees, and meet with over 100+ exhibitors.
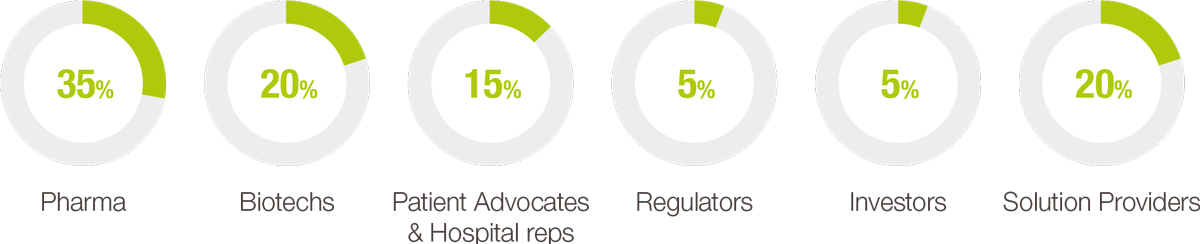
Each year, our event helps foster key collaborations for rare disease patients and orphan drug developers. The leading pharmaceutical and biotechnology companies, regulators, patient advocacy groups, payers, investors, and solution providers, all rely on the congress to convene and brainstorm ways to advance orphan drug development and improve access to life-saving therapies for rare disease patients.
Our team is looking forward to seeing you in Boston, MA on June 9-11, 2025 for another industry defining event.
TACKLING THE CHALLENGES OF
ORPHAN DRUGS TODAY
ACCESS TO TREATMENTS
Even if an orphan drug is approved, patients may not have access to due to high costs or lack of insurance coverage. Additionally, many rare disease patients require complex and expensive care, which can be difficult to access and afford.
REGULATORY CHALLENGES
The regulatory pathway for orphan drugs is often complex and lengthy, which can delay the approval and availability of new treatments. Additionally, there is a lack of standardization among regulatory agencies, which can make it difficult for pharmaceutical companies to navigate the process in different regions of the world.
LACK OF BIOMARKERS AND NATURAL HISTORY DATA
Biomarkers and natural history data are critical for understanding rare diseases and developing effective treatments. However, these are often lacking for many rare diseases, which can make it difficult to diagnose and treat patients.
NEW LEGISLATION IN THE US AND BEYOND
With the Inflation Reduction Act set to impact rare diseases, it is critical to understand the newest pathways forward. Coupled with the European Commission’s proposal to change the orphan legislation, every stakeholder involved in orphan drugs will be impacted.


ATTENDEE BREAKDOWN