ATTENDEES FOR 2017
Recognising the roles of rare diseases stakeholders, the
World Orphan Drug Congress Asia 2017
invites pharmas,
patient groups, government and experts to join the discussion on providing proper medical care
for rare disease patient care in the Asia Pacific region.
By Profile

Patient Groups
What can patient groups do to help ease challenges in orphan drug access? Understand industry perspective and collaborate to resolve challenges fast.

DOH/MOH
Why not subsidize orphan drugs? Share your perspective; be understood and work toward a solution.

Asian FDAs
How can you start to define rare disease? Leverage how others have defined rare disease and provide a pathway for orphan drugs to your rare disease patients.

Pharma, biopharma & biotech
Understand Asia’s market access culture and infrastructure. See how best to provide orphan drugs to rare disease patients in the region.
By Geography
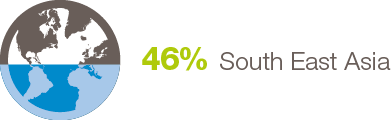
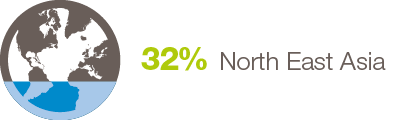
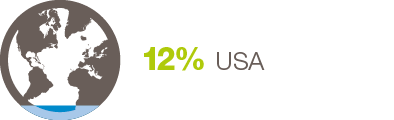


By Job Title
-
CEO
-
Chief Commercial Officer
-
Chief Medical Officer
-
VP, Rare Disease Unit
-
VP, Business Development
-
VP, Commercial
-
VP/Director, Market Access/P&R
-
VP/Director, Regulatory Affairs
-
VP/Director, Medical Affairs
-
Patient Advocate/Liaison

INTERESTED IN SPONSORING OR EXHIBITING?
Contact
Pinky Fadullon
at
pinky.fadullon@terrapinn.com
or
+65 6322 2738
