THE FUTURE OF ADVANCED THERAPIES
3,300+
Attendees
300+
Speakers
200+
Exhibitors
75+
Biotech Start-Ups
2
Days
About Advanced Therapies Congress
The Advanced Therapies Congress is Europe's largest commercial cell and gene therapy conference and exhibition. The event is for the leaders of pioneering ATMP companies and their most senior executives in charge of the latest tech and strategies that are driving the industry forward.
The conferences brings together a community of 3,300 global executives at ExCeL London, to be inspired by 300 speakers and 75 start-ups over 2 incredible days. The Advanced Therapies Congress features speakers from across the entire value chain of cell and gene therapy development; forward-thinking pharma, biotech and start- up companies, researchers, clinicians, academics, HTAs, payers and regulators.
Our Speakers
What is the Advanced Therapies Congress?
Conference

The Advanced Therapies Congress features speakers from across the entire value chain of cell and gene therapy development with 8 tracks of content over 2 days.
Exhibition

50 leading solution providers to meet your needs across all stages of cell and gene therapy development
Networking

Connect with thousands of cell and gene therapy experts in 12+ hours of built-in 1-2-1 networking
Covering the entire pipeline of ATMPs
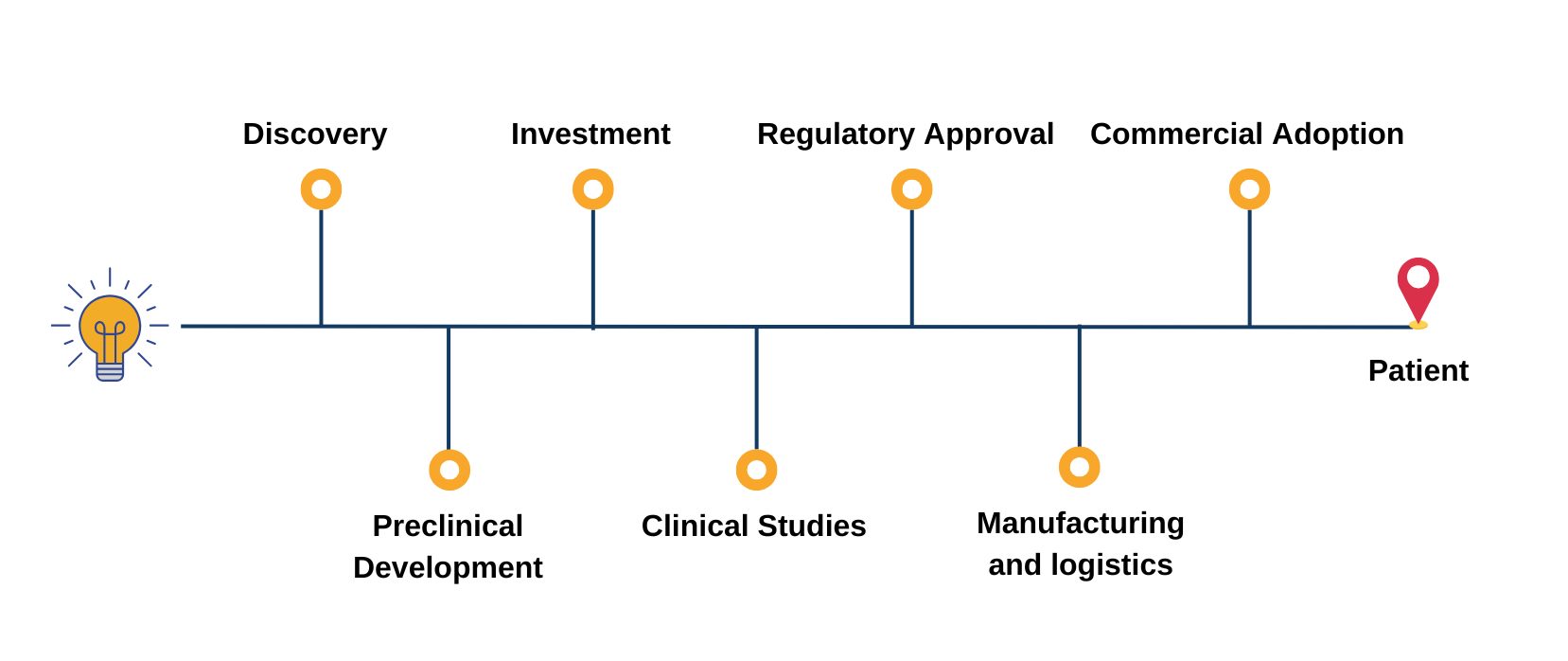
The Advanced Therapies Congress brings together speakers from across the entire value chain of cell and gene therapy development; forward-thinking pharma, biotech and startup companies, researchers, clinicians, academics, HTAs, payers, regulators.
Content Themes

"Great people and content; lots of opportunities to dialogue with experts on our sector"
– President & CEO, NKILT Therapeutics

"The most momentous leaps in CGT won’t come from solitary strides, but from a collective momentum of aligned visionaries. This conference will be a catalyst towards this goal."
– General Manager and Head of Europe, Novartis Gene Therapies

"Great mix of suppliers, manufacturers, Sponsors, exhibitors and presentations"
– Vice President of Operations, Adaptimmune

Join us in London | 17 - 18 March 2026
Get Involved with Advanced Therapies Congress