EUROPE'S LARGEST CELL & GENE THERAPY EVENT
2,500+
Attendees
300+
Speakers
100+
Exhibitors
100+
Biotech Start-Ups
2
Days
What is the Advanced Therapies Congress?
Conference

The Advanced Therapies Congress features speakers from across the entire value chain of cell and gene therapy development with 10 tracks of content over 2 days.
Exhibition

100 leading solution providers to meet your needs across all stages of cell and gene therapy development
Networking

Connect with thousands of cell and gene therapy experts in 12+ hours of built-in 1-2-1 networking
2024 Photo Gallery
Covering the entire pipeline of ATMPs
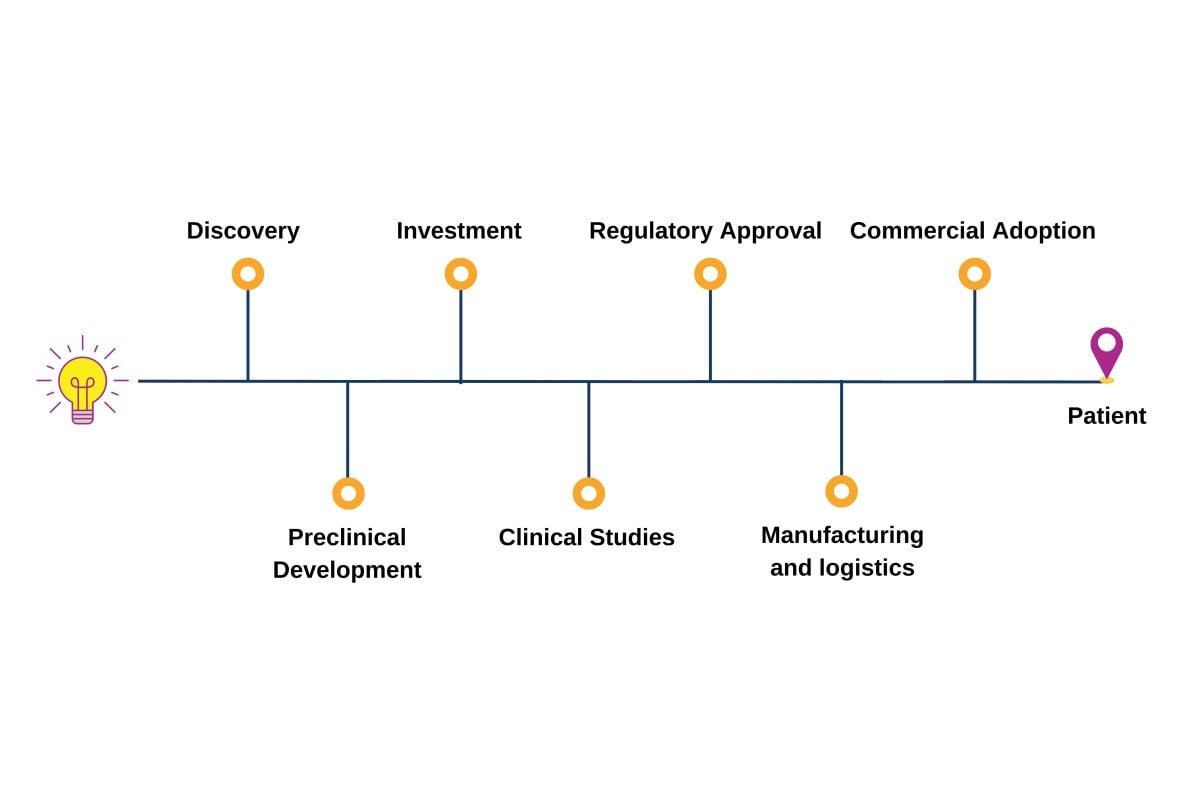
The Advanced Therapies Congress brings together speakers from across the entire value chain of cell and gene therapy development; forward-thinking pharma, biotech and startup companies, researchers, clinicians, academics, HTAs, payers, regulators.
Our incredible line-up for 2025
Essra Ridha
CMO
AVROBIO
CMO
AVROBIO

Avencia Sánchez-Mejías García
CEO
Integra Therapeutics
CEO
Integra Therapeutics

Content Themes

"Great people and content; lots of opportunities to dialogue with experts on our sector"
– President & CEO, NKILT Therapeutics

"Excellent networking activities with top level actors in the field of advanced therapies"
– Professor, University of Pisa

"Great mix of suppliers, manufacturers, Sponsors, exhibitors and presentations"
– Vice President of Operations, Adaptimmune
Join us in London | 18-19 March 2025
Get Involved with Advanced Therapies Congress






























