
15 – 17 October 2024
Hall 1, Messe Basel
Discovery • Development • Manufacturing • Fill & Finish • Market Access
Attendees
Speakers
Exhibitors
Start-Ups

The start to finish of biologic drug development from discovery through to market access across 3 days and 13 tracks

150 leading solution providers to meet your needs across discovery, development, manufacturing, fill finish and market access

Connect with thousands of biologics experts in 12+ hours of built-in 1-2-1 networking
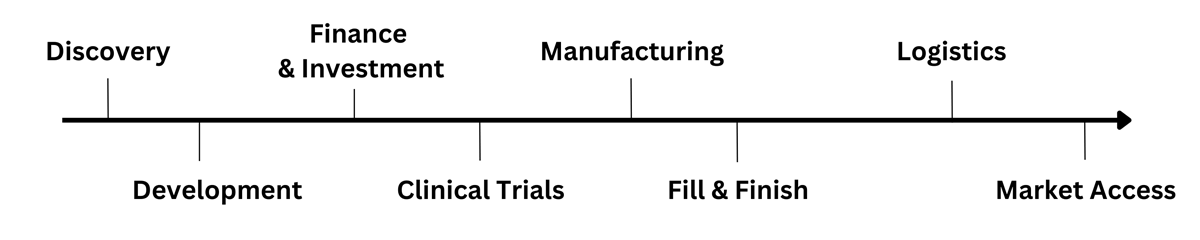
The Festival of Biologics brings together pharma & biotech, academics, research institutes, regulators, patients groups and payers together with their partners across the value chain to bring you from discovery to market.



